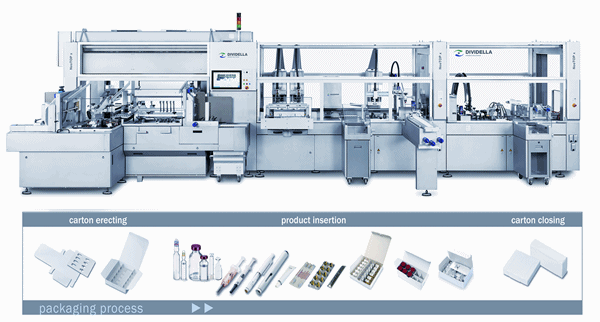
Overview
Modularity is built into every aspect of Dividella’s packaging and cartoning products and services, from the design of NeoTOP machinery to its wide range of packaging solutions.
For Dividella and its clients, the future is modular: providing easy upgrade and expansion paths and a wider range of innovative solutions on materials and packaging.
Modular design has always been a core characteristic of Dividella’s NeoTOP cartoners and top loading machinery, enhancing service life, upgradeability and flexibility to minimise Total Cost of Ownership (TCO).
Similarly, an integrated range of complementary packaging modules allows clients to adopt more space and cost-effective solutions and a wider range of materials to reduce Total Cost of Package (TCP).
Dividella is itself part of a modular ecosystem of related skills and competences within the Medipak Systems group, along with Fargo Automation, Mediseal, Seidenader, Rondo and Werum IT Solutions.
These sister companies are each highly expert in their respective core areas, able to collaborate with each other to provide complementary modules of innovation and specialised capability.
Each of these modules is becoming increasingly relevant to questions of how the pharma industry can generate sustainable competitive advantages within the Industry 4.0 concept that leverages the very latest information and communications thinking to generate innovation and progress.
A modular approach to Industry 4.0 allows Dividella to offer leading edge solutions in smart packaging, smart devices, Condition Monitoring and Predictive Analytics, Plug & Produce Internet of Things (IoT) functionalities and Enterprise Manufacturing Intelligence (EMI).
The modular philosophy
The core meaning of ‘module’ is a self-contained unit or item, which itself performs a defined task or purpose, but which can be linked with other modules to form a larger system. The modular approach has three key advantages:
- It makes it easier to configure a system to an exactly defined purpose with minimal redundancy of components or capability
- It makes it easier to expand or upgrade the system to incorporate new functions, capabilities or technologies, with minimum material waste
- It makes it easier to adopt a systemic overview across facilities and process, allowing faster identification of bottlenecks and priority areas for further investment.
However, to maximise these key advantages means overcoming the principal challenges of modularity: interconnection, interoperability and compatibility. For modularity to work to best effect, it is vital that each module actually connects properly with neighbouring modules, that each module forms its own discrete ‘centre of excellence’ without needlessly duplicating the functions of others and that each module can match the capabilities of the system. Otherwise, there is the damage that the whole system will only perform at the speed of ‘the slowest ship in the convoy’.

Modularity in top loading
Modular design and construction allows Dividella’s NeoTOP family of TOPLoader machines to form a continuous upgrade path from manual packaging of small lots up to 100,000 unites per year to fully automated high speed production of more than 24 million packs annually
The five machine family ranges from NeoTOPx, designed for semi automated packaging of small batches of blisters, ampoules, vials, syringes, injectors and similar products, through to the flagship NeoTOP 804 designed for fully automated high speed production of very large lots.
Across the range, there is consistent sharing of specialised modules that add specific capabilities, such as tailored infeeding.
The modular concept allows NeoTOP machines to expand at any time to integrate, for example, different product inserters or a manual insertion stage I the process.
The NeoTOP concept is adaptable to accommodate extreme product changes and complex pack arrangements.
Modularity in packaging
Dividella’s TOPLoading packaging solutions for pharmaceutical products follow a similarly modular philosophy emphasising a component-based approach to design, assembly and regulatory compliance.
These features include provision of flat blanks for cartons and partitions allowing printing on all sides, safe automated erecting of packs and a safer loading process, enabling 100 per cent verification after loading.
This approach offers a variety of advantages for pharma companies and their customers, influencing the complete production and logistical process and allowing a wider choice of materials. All these have positive effects on TCO and TCP.
It also allows Dividella to incorporate innovative concepts like folding ’wing’ format, extended fifth panel flap, integrated partitioning, external tamper evident wafer seals, use of 100 per cent recyclable material and space-saving designs that minimise footprint and logistics costs.
In turn these deliver further cost-saving benefits that include
- Distribution improvements: economies in warehouse storage
- Cold-chain storage and distribution savings: Reduction in package volume easing logistics burden of refrigerated truck and internal cold-chain storage space
- Damage reduction: Specifically tailored partitioned design prevent breakage and reduce risks to container closure integrity
- Packing/ Processing efficiencies: Easier tamper evident sealing for improved overall equipment effectiveness (OEE).
- Material cost savings: Elimination of plastic-based blister trays and lids can result in over 400 per cent reduction in material unit costs.
Pharma 4.0 modularity
Dividella is working in tandem with its fellow Medipak Systems companies in their respective core areas to find answers to the question of how the pharma industry can generate sustainable competitive advantages with the aid of Industry 4.0 concepts.
They are developing a modular family of advanced solutions that can work in isolation or in combination as part of a Pharma 4.0 approach.
These solutions include:
Smart packaging
Smart packaging takes product personalisation and product security to a new level, envisaging packs that communicate with the patient and with the machines in the production process. Using digitally encoded data within the package can revolutionise information and service options for providers and end-users of pharma products, such as: digital patient information leaflets, audio patient information leaflets, digital tamper-proof protection, health management, intake reminder and automatic repeat order. In production, smart packaging can instruct the machine to vary settings to the requirements of the individual package and even an individual end user.
Smart devices
Smart control devices provide the right information at the right time and place, enabling machine operators or production managers to operate and monitor the machine or system, even remotely. By means of the mobile, ‘extended’ HMI, the machine operator gains significant freedom of movement can perform tasks more efficiently, resulting in higher quality and hugely simplified changeover, setup or maintenance.
Condition monitoring and predictive analytics
Condition monitoring and predictive analytics can reduce downtimes and optimise deployment of personnel and resources by collecting data in real time but interpreting it more meaningfully to detect critical incidents before they occur and schedule preventative maintenance. Reactive or preventive maintenance is replaced by using data mining, modelling, statistics and machine learning
Plug and produce
Plug and produce lays the basis for IoT functionalities by using standardised interfaces to allow vertical integration between MES, automation and control systems. Like connecting an electronic device via a USB interface, it should be possible in the future to link a line, a system or a machine such as a packaging machine to the network, simply and straightforwardly: Plug & Produce.
Enterprise Manufacturing Intelligence (EMI)
EMI can improve product quality (process stability) and productivity (process efficiency) by translating production data into usable information for decision making. The customer can make well-founded decisions that improve process stability and process efficiency, thereby increasing product quality and productivity. Production can be supervised in virtually real time and can be continuously verified.
Target sectors
- Pre-clinical research
- Drug discovery
- Drug delivery
- Clinical trials and studies
- CRO, CMO, CRAMS & CDMO
- Formulation and ingredients
- RA & compliance
- Manufacturing and production
ACE technologies is an official representative of Dividella (KORBER SOLUTIONS) in India.
Contact Details:
E-mail: acetechnologies@vsnl.net