The Access to Medicine Foundation has warned that COVID-19 and drug resistance are threatening to derail the progress made against the HIV, malaria and TB epidemics. This new publication sets out why, with the last mile in sight, greater momentum is needed to develop new child-friendly medicines for these diseases and to rapidly make them widely accessible.
As the COVID-19 pandemic deepens, it disrupts health systems bringing prevention and treatments programmes to a halt. These disruptions are predicted to lead to a spike in deaths from HIV, malaria and TB. Models predict twice as many deaths from malaria in sub-Saharan Africa in 2020 as in 2018; an additional 1.4 million TB deaths over the next five years globally; and significant increases in new HIV infections in children, ranging from 37 per cent in Mozambique to 104 per cent in Uganda. The pandemic also risks accelerating the rate at which drug resistance is emerging.
Last mile in sight
HIV, malaria and TB pose the greatest risk to children, especially those living in low- and middle-income countries. Termed the ‘Big 3′ for their combined impact on human health, these infectious diseases have long been the target of international collaboration and funding. Nevertheless, they still account for over half a million child deaths each year, mostly in children under the age of five.
“After decades of investment, the last mile is in sight. To continue the fight against these diseases, while also battling COVID-19 and drug-resistance, children urgently need child-sized medicines. Yet they are waiting last in line for life-saving treatments,” says Jayasree K. Iyer, Executive Director of the Access to Medicine Foundation. “These timelines can be shortened with an ‘all hands-on deck’ response involving the pharmaceutical industry, regulators, governments, NGOs and civil society.”
Birds-eye view of pharma engagement
The Access to Medicine Foundation has now published a birds-eye view of pharmaceutical industry engagement with this issue. Its new series of articles maps current paediatric pipelines and product gaps for children with HIV, malaria and TB, underscoring the impact that these diseases have on children and highlighting the challenges that threaten progress. The articles identify nine case studies of real action from pharmaceutical companies and organisations working towards the delivery of these much-needed paediatric treatments.
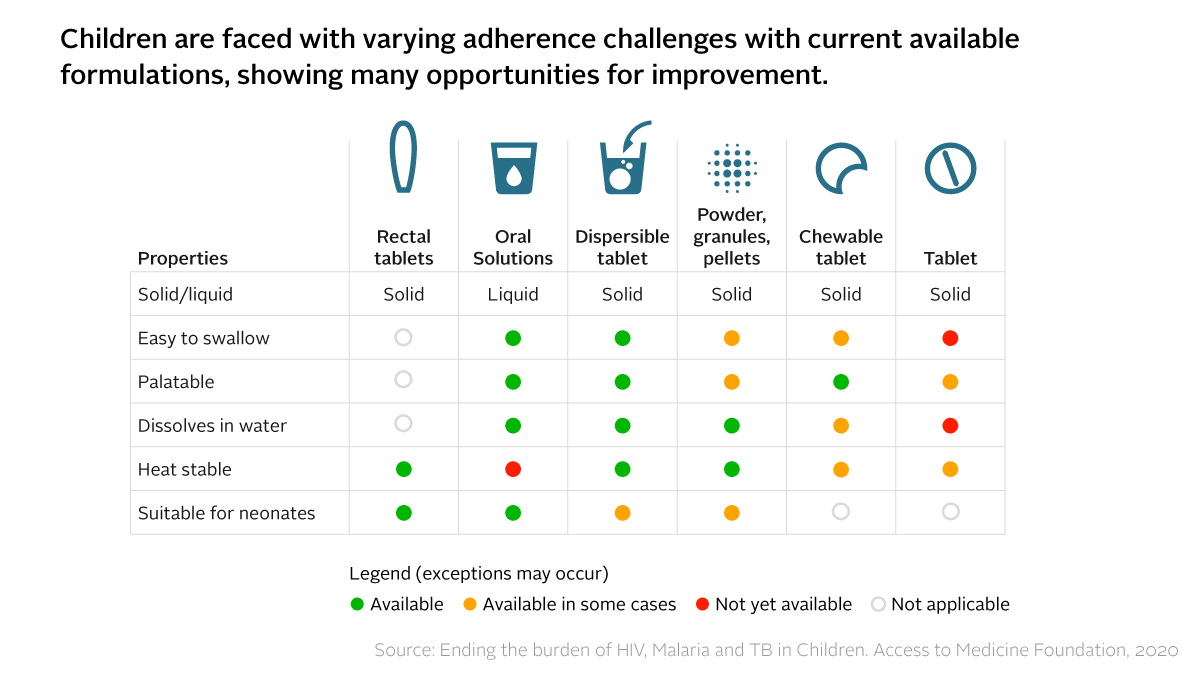
For many children, their HIV, malaria or TB medicine comes in hard pills or bitter syrups that are difficult to swallow. Caregivers often resort to breaking or crushing adult versions of medicine. This increases the risk of children not getting enough of the active ingredient with each dose, keeping them sicker for longer and exposing them to drug resistance.
It takes considerably longer for child-friendly medicines to reach the market than adult versions. This is in part due to clinical and technical challenges, but also because small and fragmented paediatric markets offer little incentive for pharmaceutical companies to invest. Even once new options are developed, children face long delays before gaining access.
New analysis of the challenges
The three articles each dive into the current situation for one of the three diseases, setting out which new child-friendly treatments are needed and specific actions for ensuring their availability.
HIV: drug resistance increases need for new child-friendly treatments
The article on HIV covers how the number of new paediatric HIV infections has decreased significantly in recent years. While this is an extremely positive trend, it also means that the market for child friendly ARVs is shrinking, making it less attractive for pharmaceutical companies to invest in developing new ones. With one in two newly diagnosed children showing resistance to commonly used ARVs, new treatments that are child-friendly are needed to replace those that are no longer effective.
Malaria: High-quality child-friendly antimalarials are needed
The article on malaria highlights how, although children under five are most vulnerable to malaria, the availability of child-friendly antimalarials is limited. In the meantime, suboptimal treatments or even substandard and falsified antimalarials medicines are prevalent, which increases the threat of drug resistance. The development and delivery of high-quality, optimal formulations for children are urgently required.
TB: New incentives and priorities are needed to direct focus on MDR-TB
In the article on TB, adherence issues are highlighted as a particular challenge for children with multidrug-resistant TB (MDR-TB), as treatment regimens can be long and grueling. Yet poor adherence also drives drug resistance. Sustained collaborative action between public and private actors will be essential to ensure access to much-needed MDR-TB medicines for children.
Safeguarding progress
While each of the three epidemics pose unique challenges, common areas of action have been identified as a basis for scaling up progress. The series of articles published today makes clear that this a shared responsibility between the pharmaceutical industry, regulators and governments.
“We must always put vulnerable populations front and centre in our global response to infectious diseases – this includes children living in low- and middle-income countries. That’s how we will finish the fight against such diseases, whether new threats such as COVID19, or endemic ones such as HIV, malaria and TB,” says Iyer.

